The Hazard Analysis and Critical Control Point (HACCP) system provides a systematic, globally-recognized approach to reducing safety hazards in food production systems.
Use a HACCP system to control potential biological, physical, and chemical hazards that threaten the integrity of each of your food products. Each of your products or processes requires its own HACCP plan.
We take you step-by-step through the process of creating and filling in a HACCP plan template below.
Complete Your Relevant Prerequisite Programs
Every HACCP plan requires a series of prerequisite programs to complete before you embark on creating the HACCP system itself.
These programs vary according to the type and way you process food. They usually include items such as:
- Water and ice safety
- Food-contact surface cleanliness and conditions
- Preventing cross-contamination
- Maintaining hygiene facilities (handwashing, toilet facilities, etc.)
- Labeling and storage programs
- Pest prevention
- Allergen management
- Training
- Waste disposal
- Temperature control
These programs form the foundation of your HACCP plan.
Nominate Your HACCP Team
The HACCP system is a team effort, and your HACCP plan is only as strong as your team.

Each HACCP team member should come from a different department to provide a well-rounded approach.
It should also include staff from the operating floor. They add practical insight into the process and help identify any oversights. Including these team members also adds a sense of ownership to the HACCP process, which makes its implementation more likely to be a success.
Complete Your Plan Template
With your team in place, you’re ready to begin working on your HACCP plan template.
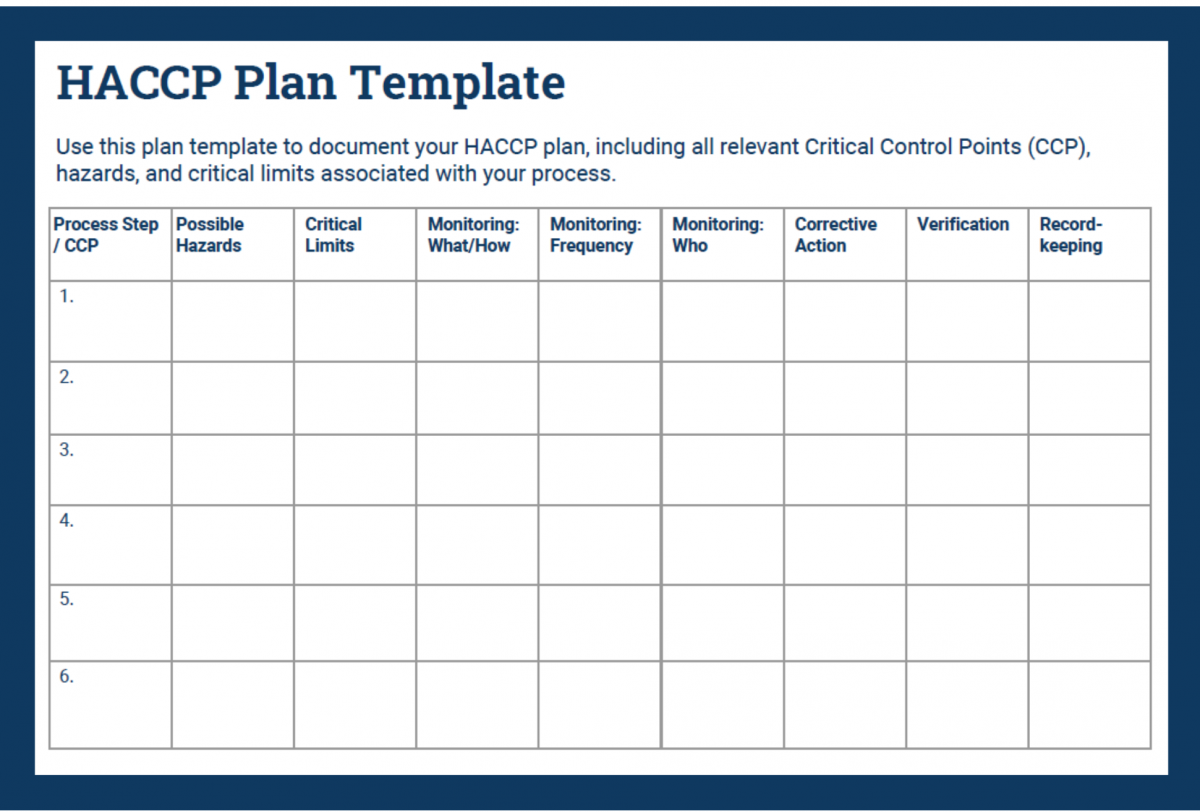
Complete your HACCP plan step by step using the following guide.
Tips for Writing Your HACCP Plan
To write your HACCP plan, keep descriptions accurate but short. Use straightforward, no-frills language. Your plan should be easy to understand and follow.
After your first draft is complete, review it and remove any information that’s not essential.
Write Your Product Description
Every team’s first task is to write a product description.

The description should be general and include:
- The food
- Ingredients
- Processing methods
- Packaging
- Storage
- Distribution procedures (frozen, refrigerated, ambient temperature, etc.)
- Example of the label and labeling information
Describe Your Intended Use and Primary Market
Who buys the food and why? This section of your product description will provide useful information for determining critical limits later on.
Many foods can be described as catering to the general public who buy the food to cook it at home. However, if you create a niche product, such as dairy-based baby formula, then you need to identify them specifically.
For example, to write a description of baby formula, you might include that the formula is designed to be mixed with water.
Develop Your Commodity Flow Chart
The second step of your template requires you to identify the scope and the process or commodity flow.
Here, the HACCP team uses the product description, intended use, and primary market to develop a complete description of the production process from start to end.
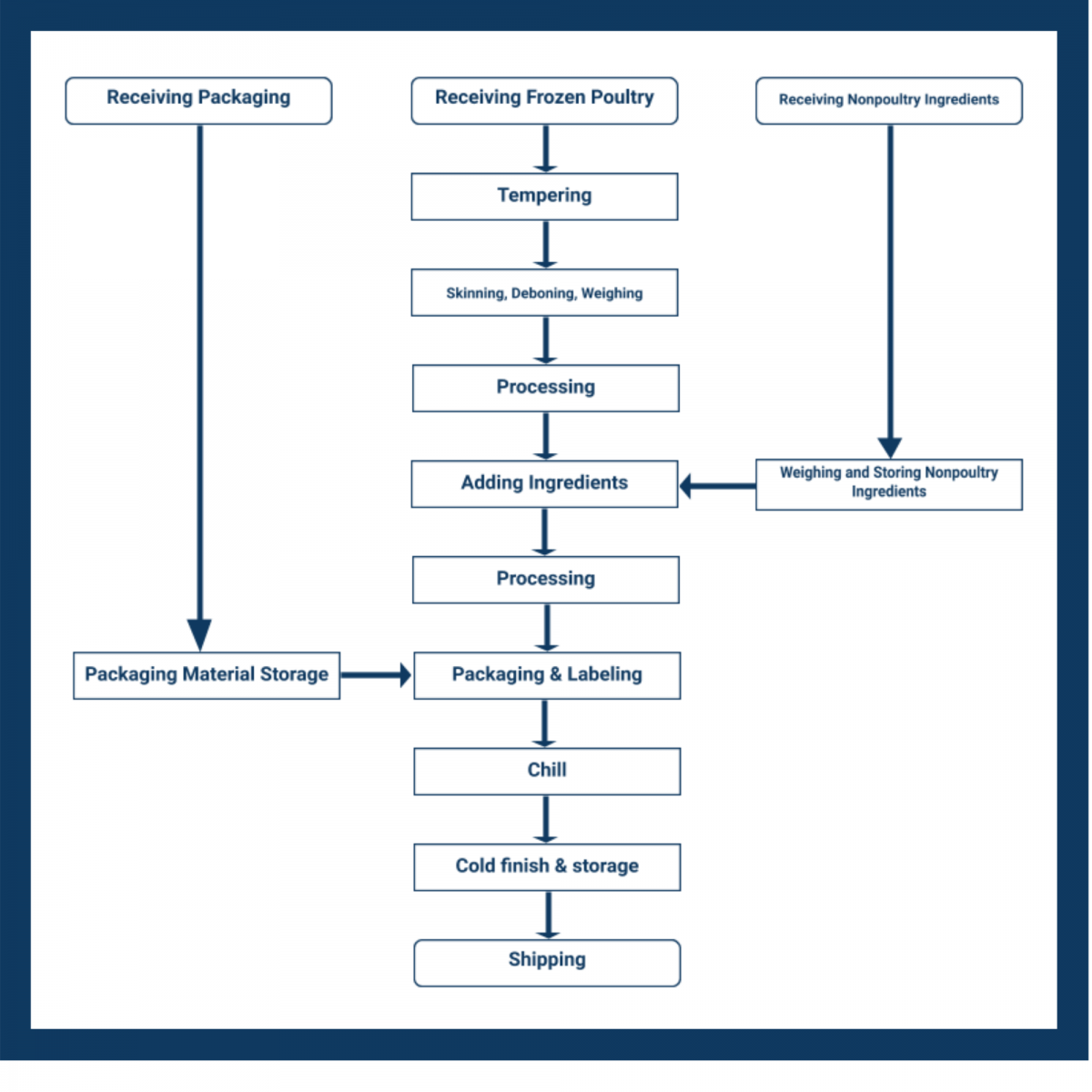
The commodity flow diagram, or flowchart, simplifies the process steps. It doesn’t need to include fine details at this point in the diagram — that comes later.
However, there are some common oversights. Make sure that your list of process steps also includes:
- Any inputs
- Intended delays
- Procedures that vary by shift
- Return of product to the process
- Any and all outputs from the product
Verify the Flow Chart
The next step is to verify the flow diagram that you just created. Your team needs to give it more than a once over: you should visit the site where the system takes place and walk through each step on the chart as you would during the production process (i.e., walk the line).
Your verification step ensures that the flow chart includes all practices and materials in the process so that you can identify every single relevant control point in the following step.
If the process changes across shifts or seasons, then your HACCP team should visit during each version of the process at a minimum. Multiple visits help to create more complete flow diagrams.
Remember: if your commodity flow diagram is incorrect, then your HACCP plan will be ineffective. Take the time to verify and get it right the first time.
Run a Hazard Analysis
With the process flowchart complete, your next step is to evaluate it for hazards. Examine each step you identified in your flowchart for biological, chemical, and/or environmental risks.
The hazard analysis will help you determine which steps in your commodity flow are critical in protecting consumers. (See Principle 1 for more information.)
Document hazard type, likelihood, and control methods on a hazard analysis worksheet. Be sure to note whether or not you have legal requirements for controlling each of the hazards you identified.
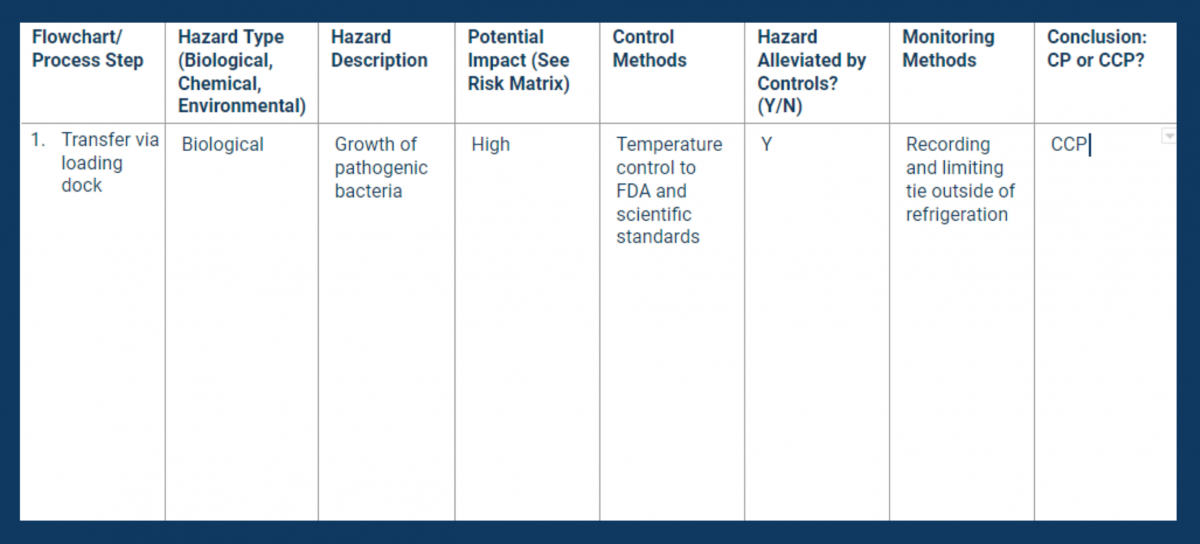
Use a second tool — the CCP decision tree — to help you identify the relevant CCPs.
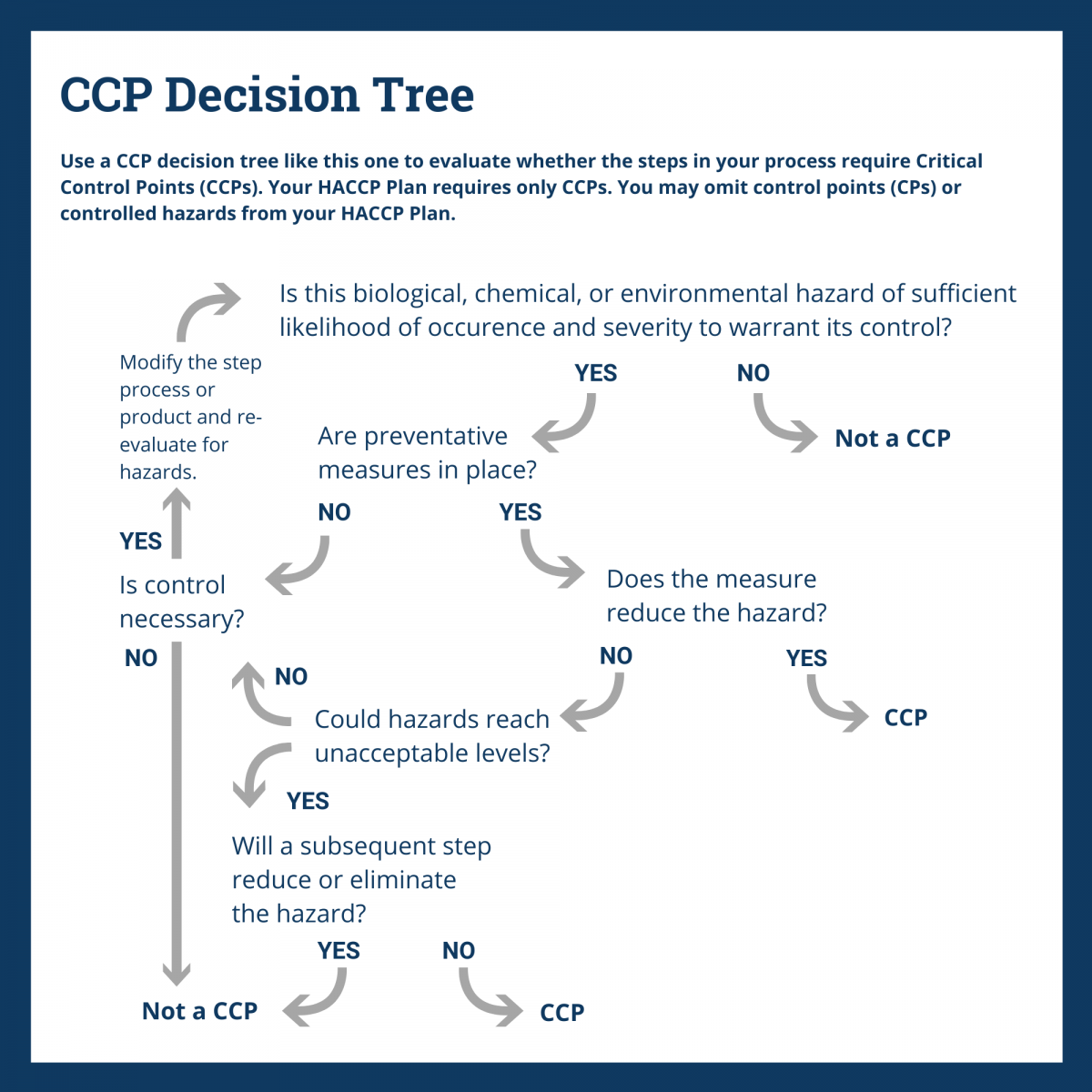
Identify Critical Control Points
With your process flow verified and hazards identified, you are ready to use a CCP Decision Tree to identify the critical control points (CCPs) for each process step. You’ll find this explained in Principle 2.
A CCP is a point at which you must intervene to either eliminate, reduce, or prevent a hazard to an acceptable limit.
It’s important to remember that one CCP may control more than one hazard and you may need more than one CCP to control a single hazard.
In your HACCP template, fill in the first column with your CCPs. Place them in consecutive order.
Next, fill in the second column with the hazards you identified in your analysis.
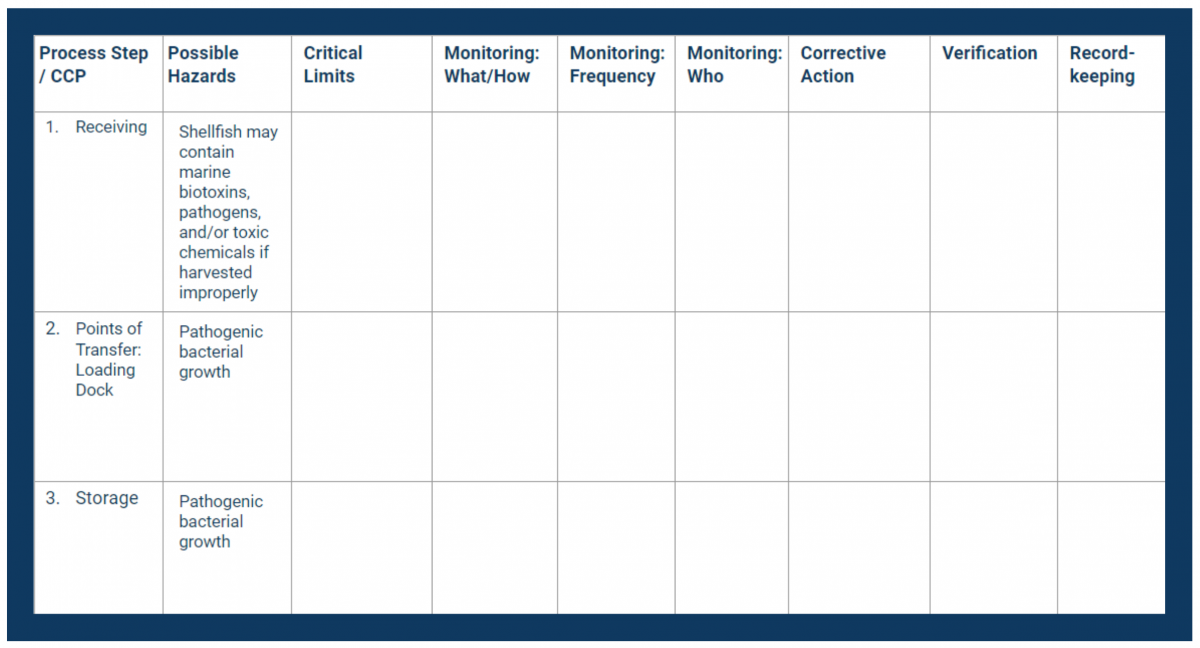
Set Critical Limits for Each CCP
The critical limit (see Principle 3) represents the value (whether highest or lowest) that is acceptable for food safety.
If the values you’re monitoring fall outside of the critical limit, you face a greater risk to consumer health. Therefore, each critical limit should be as strict as any legal limits that apply to your processes, if not more so.
In your HACCP plan, fill in the critical limits column with measurable controls.
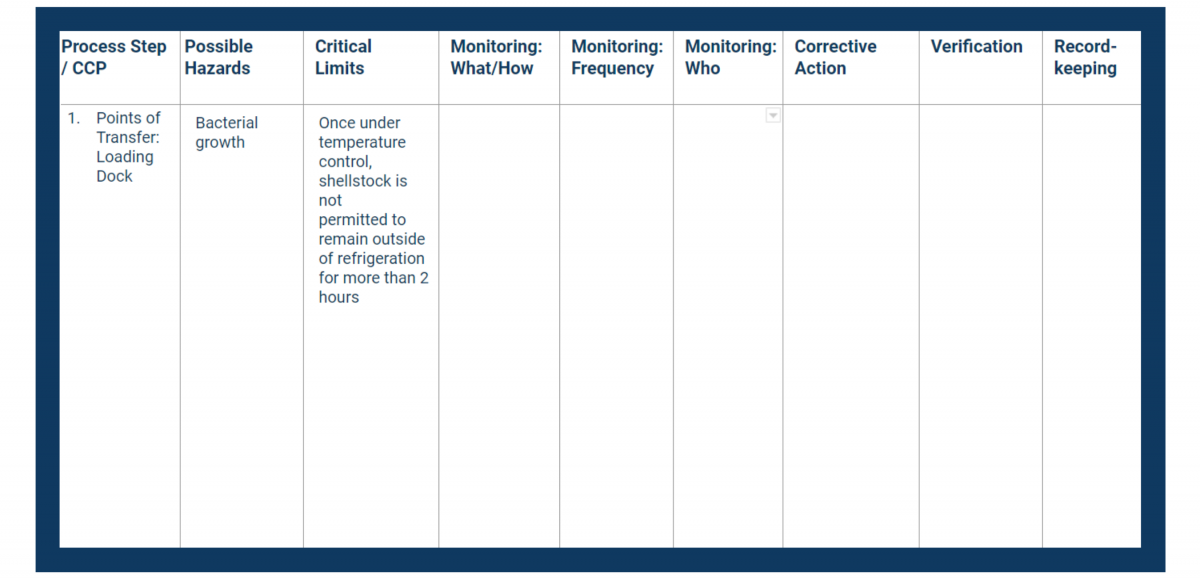
You’ll also need to validate your critical limits as well as monitoring and corrective actions both after completing your HACCP plan and periodically as long as the HACCP plan remains in place.
Identify Monitoring Procedures for Each CCP
HACCP Principle 4 covers the establishment of monitoring procedures for CCPs.
Monitoring is a planned sequence of measurements and observations that determine whether the CCP remains under control. It’s also an important part of recordkeeping and future verification.
Your monitoring procedure helps identify trends towards loss of control, which allows you to use the next step (corrective actions) to avoid deviating from the critical limit.
When you identify the monitoring procedures, you must:
- Nominate a person to conduct the monitoring (must be a specific individual)
- Determine what to monitor
- Identify the process for monitoring
- Create a process timeline (when it will be monitored, frequency, etc.)
- Nominate a person to audit the monitoring system
As you create the processes, you must consider issues like the number of critical control points involved as well as the preventive measures to be taken and the complexity of the monitoring.
Document all of the above in the monitoring columns of your HACCP plan.
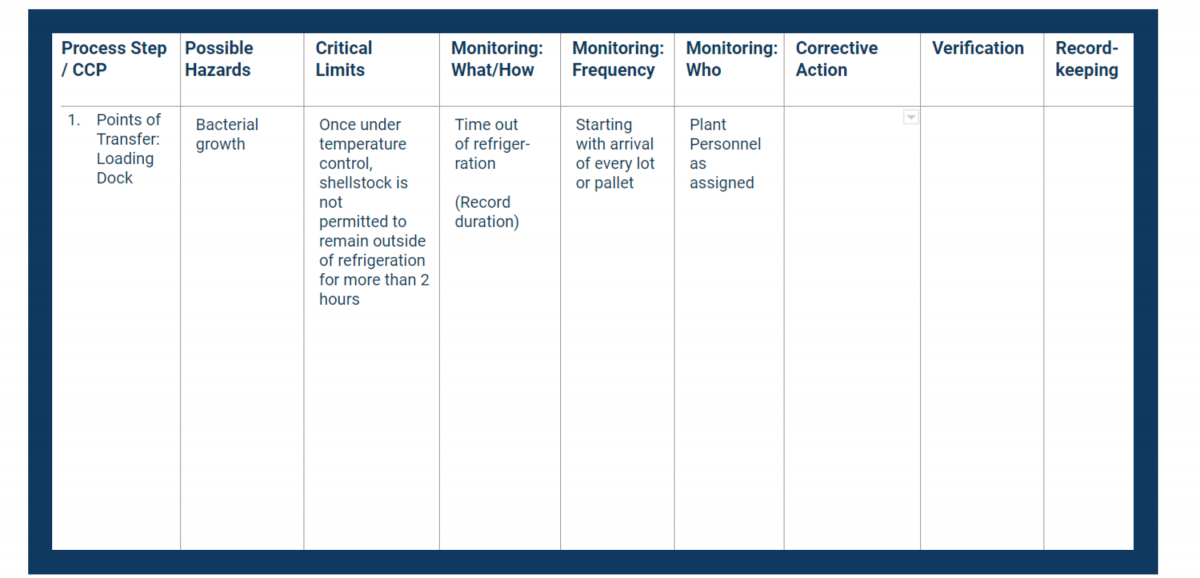
Keep in mind that responsible staff needs to be trained in the monitoring technique and have a keen understanding of the purpose of the process.
Ideally, they will be in a position to provide unbiased reporting to ensure accuracy. Staff should never check their own work.
Define Corrective Actions for Each CCP
Principle 5 requires you to identify corrective actions for any problems that occur in each case.
Corrective actions do three things:
- Restore control
- Deal with product impacted by the loss of control
- Investigate the cause of the loss of control
In addition to determining your corrective actions, you also need to identify and note:
- Who is responsible for what corrective action
- What information must be recorded, where to record it, and who should record it
- Who will audit the corrective action and double-check the data recorded
For example, if you work in meat production, then legislation is likely to require items like only admitting clean and healthy animals for slaughter and dressing.
Use the Corrective Action column in your HACCP plan to document critical procedures for controlling breaches.
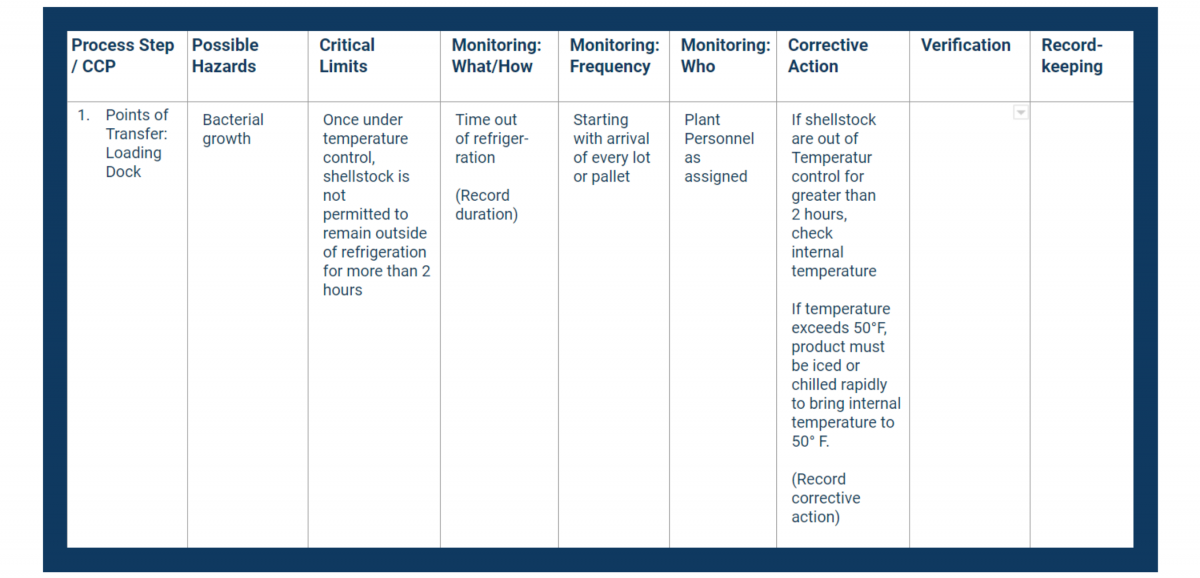
Validate and Verify
Each of the plans developed at your organization requires verification and validation before you confirm them as ready for use on the floor. These are described in Principle 6.
It consists of two activities. First, you verify that the HACCP plan will work as designed. This requires on-site work.
Second, validation uses scientific and technical principles to identify whether the HACCP plan offers the control needed for identified hazards.
The same rule applies here as with monitoring: validation and verification should be done either by outside individuals or team members who won’t participate in daily monitoring or data collection.
However, the initial validation step is only the beginning.
Your HACCP plan should also identify future verification and validation points. Both the frequency and method should appear in the plan.
In the verification column, record how you will stay on top of your processes and records.
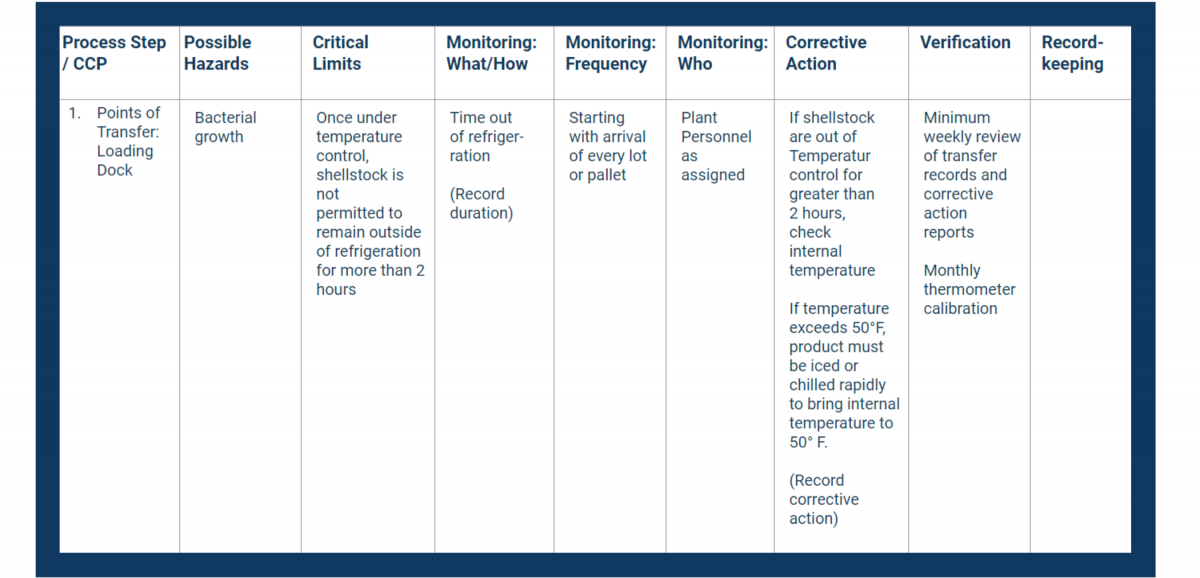
Verification usually includes address these items at a minimum:
- Pre-requisite programs
- CCPs
- Calibration
- The HACCP Plan
Validation should occur at least once a year, and it may include items such as:
- Hazard analysis justification
- Support of critical limits
- Support for monitoring activities
- Support for hazard identification
- Support for CCP location
Verification and validation are important for all businesses, but they become critical for those monitored by governments.
For example, if you run a meat or poultry operation under the purview of the USDA, then you will find that USDA inspectors run some verification activities. Both programs are required by the USDA.
Identify Logs and Records
Finally, identify the required records and create systems that keep them up to date (Principle 7). Both your team and regulators require this.
You’ll need to carefully record the key activities associated with your HACCP plan, including:
- Critical limit monitoring logs
- Testing and calibration logs
- Corrective action log
- Verification logs
Place all necessary recordkeeping activities in the final column of your HACCP plan.
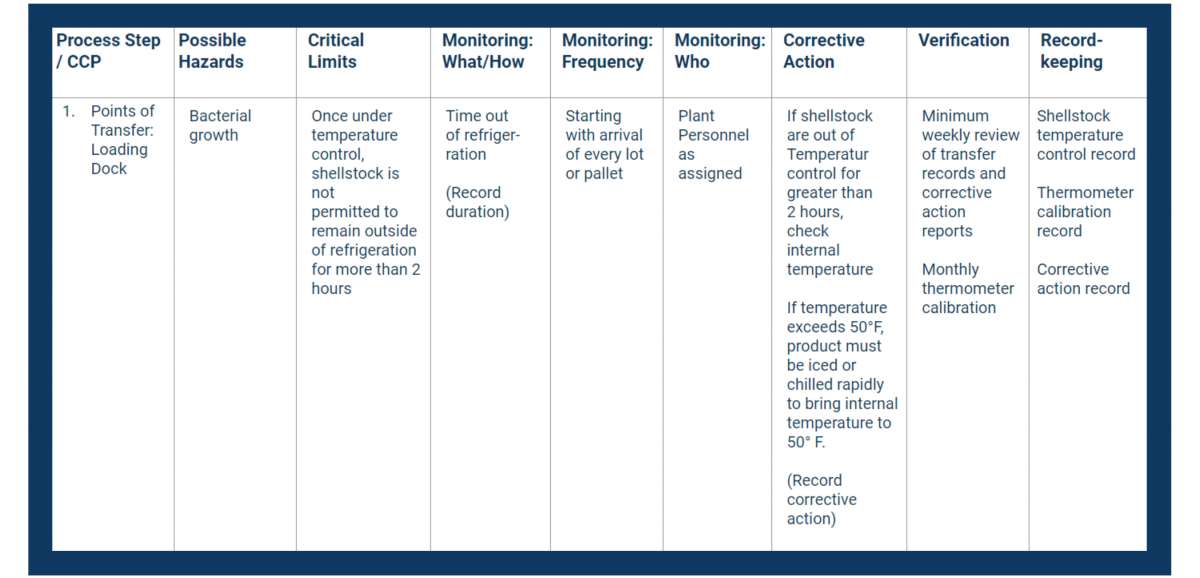
Train your team to document and store their HACCP-related activities.
Without consistent paper or digital logs, you lose critical information regarding your product and, if a deviation leads to consequences, you may be subject to penalties.
Double-Check Your Documentation
To wrap everything up, bundle your HACCP plan template with the other documents relevant to your system:
- HACCP plan documents (HACCP team, plan template, product description, and product flowchart)
- Hazard analysis
- Explanatory notes
- Monitoring arrangements
- Validation and verification arrangements
- Policy documents (hygiene, monitoring, corrective action)
It’s helpful to keep paperwork simple. Then, it will be easy to read, complete, and keep updated. Each record should also identify the person in charge of that record.
Become a HACCP Master
Finally, your HACCP plan is complete — but it’s only as good as your implementation. Your HACCP system helps you monitor critical control points to prevent a slide towards chaos.
It’s important not to underestimate the value of verification and validation throughout the process as well as the importance of your paperwork. These are critical for regulatory compliance and certification. More importantly, they help you ensure that your system works as designed.
Use the following templates to complete your HACCP Plan:
- HACCP Plan Template
- Product Description Template
- Process Flowchart Template
- CCP Decision Tree
- Hazard Analysis Worksheet
- HACCP Plan Audit Checklist
- More HACCP Checklists
Don’t forget: digital recordkeeping and data management software can simplify your HACCP system upkeep and make compliance more manageable.
